- Water Balance Calculation for Sulfuric Acid Production Plant from Sulfur (Part One
1. The source of water for the sulfuric acid production unit from sulfur
1) Moisture brought in by air
This part of the water is usually absorbed into the system by a drying tower with about 98% concentrated sulfuric acid. The moisture content in the air varies significantly due to different regions, temperatures and air humidity. The total amount of water is also closely related to the concentration of the gas controlled by the device. The amount of water added varies greatly and is basically a difficult point to control in the entire sulfur-to-acid system.
2) Add water to the low-temperature heat recovery section
The low-temperature heat recovery section absorbs the sulfur trioxide gas from the first rotation. Under the premise that the conversion rate of the first rotation is fixed, the acid concentration of the second stage of the low-temperature heat recovery tower is fixed, and the acid concentration of the produced acid is fixed, the water addition volume of the low-temperature heat recovery section is relatively fixed. It is the point with the most water addition volume in the sulfur acid production system and also the easiest point to control.
3) Add water to the dry suction section
Dry adsorption is responsible for the absorption of sulfur trioxide in the second conversion, while balancing the acid concentration produced by drying and acid transfer and low-temperature heat recovery. The amount of water added here is relatively small and is greatly affected by drying and acid transfer.
2. Set the scene
It is known that the local air pressure in a certain area is 97KPa, the temperature at that time is 25℃, and the humidity is 60%. There is an 800,000-ton/year sulfuric acid production unit from sulfur in this area, and the full-load control gas concentration is 11.0%. To simplify the calculation process, sulfur trioxide is not considered in the sulfur-burning furnace. The moisture content of the air at the drying outlet is set to zero, and the one-revolution conversion rate of the unit is 95%. The total conversion rate is 99.80%. This device is equipped with a low-temperature waste heat recovery system. The acid concentration of the acid produced by low-temperature heat recovery is controlled at 99.6%, and the acid concentration of the drying cycle is controlled at 98%. The remaining acid is fed into the secondary suction circulating acid tank. The liquid level of the drying acid tank is controlled by the two-suction circulating acid tank valve of the drying string.
3. Calculation of moisture content for each part
3.1 Calculation of Drying water volume
1) Calculation of air moisture content
The moisture content in the air can be calculated through different methods and formulas.
Method One: Use the concepts of absolute humidity and relative humidity. Absolute humidity refers to the mass of water vapor contained in a unit volume of air, usually expressed in grams per cubic meter (g/m³). Relative humidity refers to the percentage value of the actual water vapor density in the air to the saturated water vapor density at the same temperature. Absolute humidity can be found in the table below (the data in the table is sourced from the Internet and may differ from the actual data), while relative humidity can be obtained from the weather app on your mobile phone
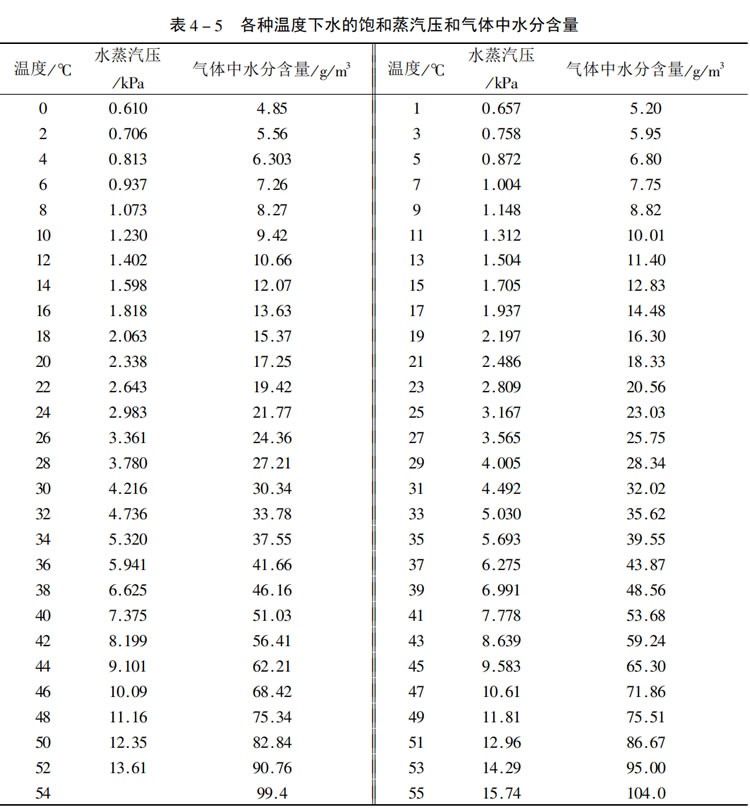
According to the above table, the absolute humidity at 25 degrees is 23.03g/m ³.
The calculation shows that the moisture content of the air is 23.03*60%=13.818g/m ³.
The gas volume required by this device under full load is 100/98/99.8%*22.4/11%= 208,208 nm3 /h.
According to the ideal gas state equation P1V1/T1=P2V2/T2,
The required gas volume under working conditions is calculated as V2=101.325*208208*(298.15)/ (97*298.15) = 217,491 m ³ /h.
The moisture brought in by the air can be calculated as 217,491 m ³ /h*13.818g/m ³ =3t/h
Method Two: Calculate using saturated vapor pressure. The formula is as follows
微信截圖_20250929163136_20250929.png)
HS----- water content in air, kg/m ³
n----- relative humidity
Ps- The saturated pressure of water at a certain temperature, Pa
P---- local atmospheric pressure at that time
It can be seen from the above table that the saturation pressure of water at 25℃ is 3167Pa
The moisture content in the air =60%*18/22.4*3167/(97000-3167)=0.01191kg/m ³
The calculable water volume brought in by air is 217,491 m ³ /h*0.01191kg/m ³ =2.6t/h
The above table shows the data found through the network. It may differ from the actual data. The calculated data is basically close at normal temperature, but there may be deviations at high temperatures.
From the above calculation, it can be seen that in a low-temperature environment, the amount of water brought into the system by the air is relatively small, and the dry and acidic concentration can be easily maintained. However, in extremely high-temperature weather, for instance, when the temperature in a certain area reaches 38℃ and the humidity reaches 80%. The absolute humidity as shown in the above table is 46.16g/m ³
Calculate the air volume as 101.325*208208*(311.15)/ (97*298.15) = 226,974 m ³ /h
The full-load air-carrying capacity of the device =46.16g/m ³ *80%* 226,974m ³ /h=8.38t/h
At this point, it is not easy to maintain the dry acid concentration, which also has a significant impact on the water balance of the entire system. We have no choice but to adopt other measures to control the acid concentration balance.
2) Calculation of acid concentration balance for drying
The air-carrying water volume is calculated based on Method one at 3t/h.
Since the drying cycle is a dynamic equilibrium process, during operation, the acid concentration in the drying tower and the liquid level in the circulating acid tank remain constant. We only need to calculate that the low-temperature heat recovery system's acid (99.6%) in the drying circulating acid tank absorbs moisture from the air and is diluted to 98% acid in the second absorption circulating acid tank.
Calculate the acid transmission volume m1*99.6%/(m1+3t/h)=98% m1=183.75t/h
According to the calculation, 183.75 tons of acid per hour produced by HRS can maintain a dry and concentrated acidity. The acid production from low-temperature heat recovery can meet the demand (the subsequent calculation shows that the acid production from low-temperature heat recovery is approximately 298 tons per hour).
Under extreme weather conditions of 38℃, the air carries 8.38 tons of water per hour
Calculate the acid transmission volume m2*99.6%/(m2+8.38t/h)=98% m2=513t/h
At this point, to maintain a dry acid concentration of 98%, the acid volume required for the drying circulation acid tank in the low-temperature heat recovery system is 513 tons per hour, which has already exceeded the total acid production from low-temperature heat recovery. We can only take measures such as reducing the drying acid concentration or using a diluter for drying to achieve this.
If all the acid produced by the low-temperature waste heat recovery system is calculated to enter the drying and circulating acid tank,
The dry acid concentration can only reach =298*99.6%/(298+8.38)=96.9%
To achieve a 98% acid concentration, it is also necessary to increase the acid production through a low-temperature heat recovery diluter in the drying string
Calculate the acid volume of the low-temperature heat recovery diluter for the drying string as (513-298) *99.6%/98%=218.5t/h
The temperature and humidity of the air are greatly influenced by the weather, especially in areas with large temperature differences between day and night and where the weather changes rapidly from sunny to cloudy. Therefore, the control of acid concentration is particularly difficult.
The calculation content is considerable, and the water balance calculation needs to be completed through several releases. For the next issue, see the calculation of low-temperature heat recovery and secondary water absorption equilibrium.


